FDA Grants Clearance To Levita Magnetics’ Surgery Platform
The MARS platform combines magnets and machinery.
Levita Magnetics' MARS platform.
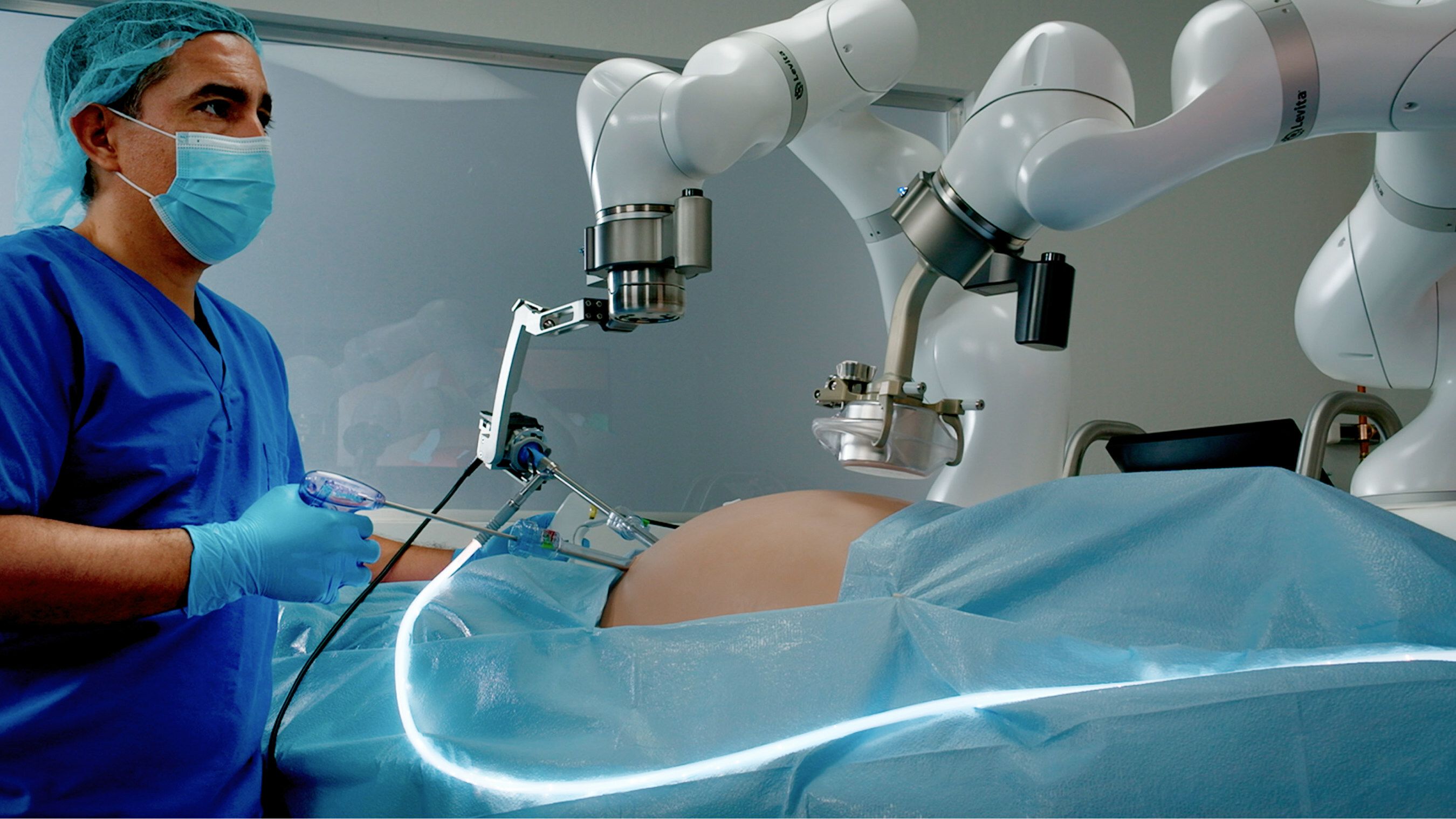
Levita Magnetics announced that it has received FDA clearance for its MARS platform.
The system is a surgical platform that uses magnets and machines to reduce the number of incisions while providing surgeons with control. The minimally invasive platform is designed for use with abdominal surgery.
In a press release, Levita Magnetics CEO Dr. Alberto Rodriguez-Navarro said, “Today marks a significant milestone in Levita's mission to provide more patients access to state-of-the-art surgical technology. MARS is poised to revolutionize surgical options for a broad range of patients. With this FDA clearance, we eagerly anticipate making a substantial impact across the value chain."
The MARS platform is designed for use in both hospitals and ambulatory surgery centers.